Mathpix Snip is constantly improving to provide users with the best image conversion experience possible, particularly when it comes to digitizing complex chemistry structures. Our updated algorithm now has the capability to recognize stereochemistry, Markush structures, and diagrams that it previously struggled with.
Stereochemistry
Stereochemistry is the study of the three-dimensional properties of molecules. Our new algorithm can now recognize stereochemistry and accurately convert it into SMILES. All the Mathpix Snip users can easily capture stereochemical diagrams and formulas using our desktop, web, or mobile app, and quickly insert the converted result into their notes and research.
Example input:
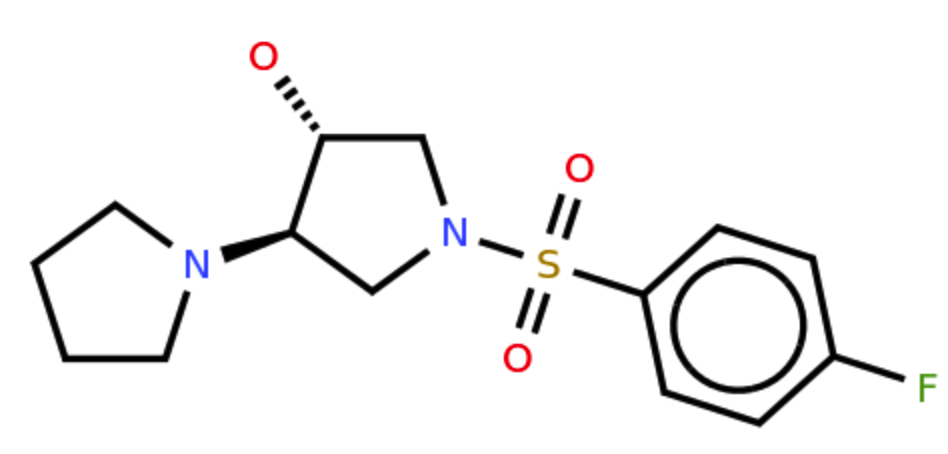
Example MMD output:
<smiles>O=S(=O)(c1ccc(F)cc1)N1C[C@@H](O)[C@H](N2CCCC2)C1</smiles>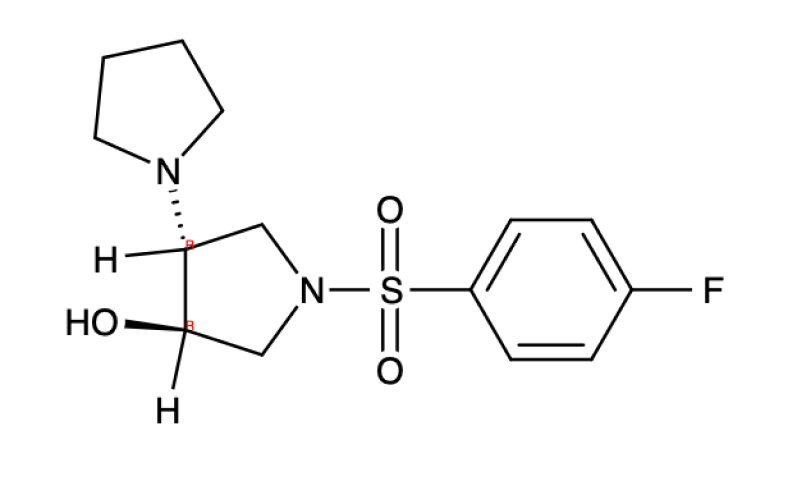
Markush Structures
Markush structures are chemical structures that contain a set of generic atoms, often used to represent a class of compounds with similar properties. Mathpix Snip Apps accurately recognize Markush structures, making it easier for chemists to identify and represent complex chemistry, as well as to use it in scientific papers.
Example input:
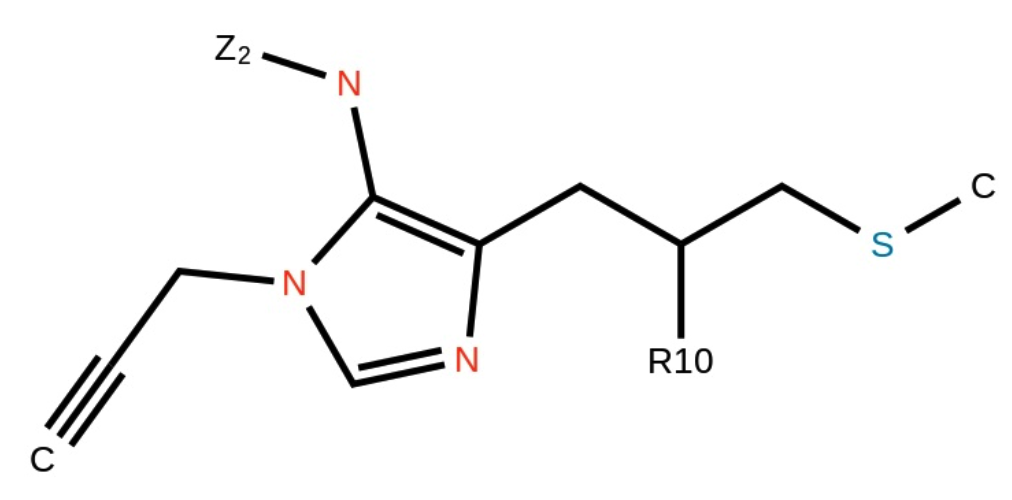
Example MMD output:
<smiles>[Z2]Nc1c(CC([R10])CSC)ncn1CC#C</smiles>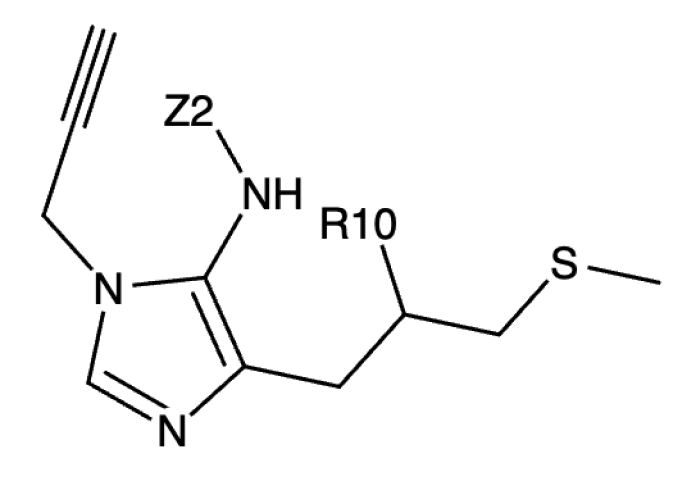
Other updates
In addition to digitizing stereochemistry and Markush structures, our updated algorithm also improves the recognition of other complex chemical structures, such as diagrams and reactions.